Model 400C Time-Resolved Electrochemical
Quartz Crystal Microbalance
The quartz crystal microbalance (QCM) is a variant of acoustic wave microsensors that are capable of
ultrasensitive mass measurements. Under favorable conditions, a typical QCM can measure a mass change of 0.1-1
ng/cm2
. QCM oscillates in a mechanically resonant shear mode under the influence of a high frequency AC electric
field which is applied across the thickness of the crystal. Figure 1b below shows an edge view of a QCM crystal
undergoing oscillatory shear distortion. The central portions of the top and bottom of the crystal are coated with a
typically disk-shaped thin metal film (e.g., gold). The mass sensitivity of the QCM originates from the dependence
of the oscillation frequency on the total mass of the metal-coated crystal, including any adlayers of deposited
materials, as given by the Sauerbrey equation:
∆f = -2f0
2 ∆m / [A sqrt(µρ)]
where f0 is the resonant frequency of the fundamental mode of the crystal, A is the area of the gold disk coated onto
the crystal, ρ is the density of the crystal (= 2.684 g/cm3
), and µ is the shear modulus of quartz (= 2.947 x 1011
g/cm.
s
2
). For example, using our crystal, which has a 7.995-MHz fundamental frequency, a net change of 1 Hz
corresponds to 1.34 ng of mass adsorbed or desorbed onto the crystal surface of an area of 0.196 cm2
.
QCM in conjunction with electrochemistry (EQCM) has been widely employed for the determination of
metals deposited onto the crystal, studies of ion-transport processes in polymer films, biosensor development, and
investigations of the kinetics of adsorption/desorption of adsorbate molecules. In EQCM experiments,
measurements of various electrochemical parameters, such as potential, current, and charge at the working electrode,
are conducted simultaneously with the acquisition of the corresponding frequency and resistance changes, using the
experimental setup shown in Figure 1a. For any model in the 400C series, application of a specific potential
waveform (e.g., triangular potential waveform for cyclic voltammetric experiments), current measurement, and
frequency counting are carried out with a potentiostat/frequency counter, which is in turn controlled by a computer.
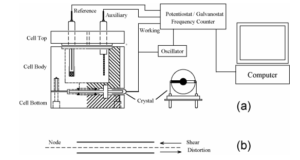
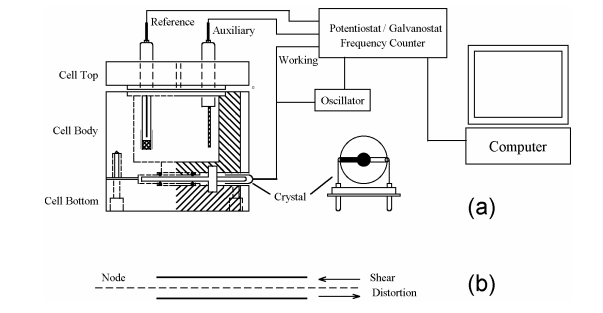
Figure 1. Schematic representation of a typical EQCM instrument. (a) The quartz crystal has a fundamental
frequency of 7.995 MHz and is coated with thin gold films on both sides. The gold disk deposited on the top side of
the crystal is in contact with the electrolyte solution and used as the working electrode. The top view of the goldcoated crystal is also shown. (b) Edge view of QCM crystal showing shear deformation. The disk thickness and
shear deformation have been exaggerated for clarity.
The 400C series contains a quartz crystal oscillator, a frequency counter, a fast digital function generator,
high-resolution and high-speed data acquisition circuitry, a potentiostat, and a galvanostat (Model 440C only). The
QCM is integrated with potentiostat and galvanostat, to facilitate simple and convenient EQCM studies. Instead of
measuring the frequency directly, the 400C series uses a time-resolved mode as follows. The observed frequency of
the QCM is subtracted from a standard reference frequency, and the resulting difference is measured by a reciprocal
4
technique, greatly reducing the required sampling time and yielding much better time resolution for the QCM signal.
With direct counting, a QCM resolution of 1 Hz requires 1 second of sampling time, 0.1 Hz resolution requires 10
seconds, etc. In contrast, our time-resolved mode allows the QCM signal to be measured in milliseconds with much
better resolution.
The potential control range of the instrument is ±10 V and the current range is ±250 mA. In addition to
QCM and EQCM measurements, the instrument is capable of a wide range of techniques, and is suitable for generalpurpose electrochemical applications. The instrument is very sensitive and very fast, capable of measuring current
down to the picoampere level. The scan rate in cyclic voltammetry can be up to 5000 V/s with a 0.1 mV potential
increment or 10000 V/s with a 1 mV potential increment.
Figure 2 shows the voltammogram of underpotential and bulk depositions of Pb from a 0.1 M HClO4
solution containing 1 mM Pb2+, and the corresponding frequency changes have been plotted as a function of the
applied potential. In Figure 2a, the cathodic peaks at –0.28 V and at ca. –0.59 V have been assigned to the
underpotential deposition of monolayer Pb and the bulk deposition of multlayers of Pb, respectively, whereas the
anodic peaks at –0.41 V and at –0.28 V are attributable to the stripping of the deposited Pb. The frequency-potential
diagram (Figure 2b) displays the frequency decrease due to the deposition of monolayer Pb (about 25 Hz or 33.5 ng
between –0.28 V and –0.59 V) and the more drastic frequency decrease arising from bulk Pb deposition (a net
change of 425 Hz or 573.8 ng at ca. –0.5 V).
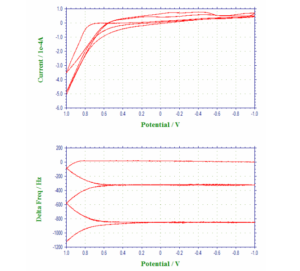
Figure 3. Voltammogram and QCM data of oxidation of pyrrole to form polypyrrole film. Scan rate 0.1 V/s.
The instrument can also be used to perform standard QCM measurements. Figure 4 shows QCM data for a
flow cell detection experiment. The total frequency change observed was less than 8 Hz, with extremely low long
term drift and noise levels..
The model 400C series is the upgrade to the model 400/400A/400B series. The new design provides more
stable and accurate potential control (1 mV, 0.02%), and it also allows the resistance change and frequency change
to be measured simultaneously.
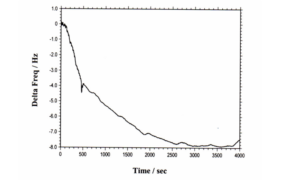
Figure 4. A typical flow injection-QCM experiment. As soon as the sample is injected, the QCM starts recording
the frequency change (t = 0). The pump is stopped at 460 s (where a small glitch on the curve can be seen). The
reaction is completed about 40 min after sample injection. The total monitoring time is over 1 hr. A net change of 8
Hz is monitored. After 40 min or so, the frequency becomes very stable again (for at least more than 20 min, the
frequency drift is much less than 1 Hz).
The 400C series has a USB port (default) and a serial port for data communication with the PC. You can
select either USB or serial (but not both) by changing a switch setting on the rear panel of the instrument.
16-bit highly stable bias circuitry is added for current or potential bias. This allows wider dynamic range in
AC measurements. It can also be used to re-zero the DC current output.
The EQCM cell consists of three round Teflon pieces (Figure 1a). The total height is 37 mm with a
diameter of 35 mm. The top piece is the cell top, which holds the reference and counter electrodes. There are also
two 2 mm holes for manual purging. The center piece is the solution cell, and the bottom piece is for mounting
purposes. Four screws are used to tighten an O-ring seal between the bottom and center pieces, with the quartz
crystal sandwiched between them. The diameter of the quartz crystal is 13.7 mm. The gold electrode diameter is 5.1
mm.

















Reviews
Clear filtersThere are no reviews yet.